Superhydrophobic Materials
What is superhydrophobic?
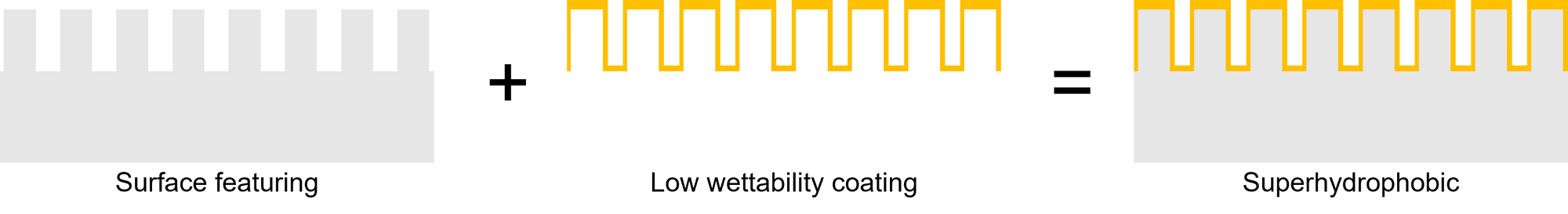
Superhydrophobic is a concept that the surface has extremely low wettability, which is quantified by the apparent contact angle θa. Superhydrophobic material is composed of the surface featuring and low-wettability coating, such as Teflon. It is because the air trap inside the surface feature prevents water penetration, so the solid-liquid interface is reduced. According to Cassie model1 , the θa is equal to the equation:
cos θa = φ(cosθ +1)-1.
Given that φ is the solid fraction, and θ is the intrinsic contact angle. The smaller solid fraction leads to a higher apparent contact angle. We define a superhydrophobic surface if the apparent contact angle is above 1500 .
Why superhydrophobic?
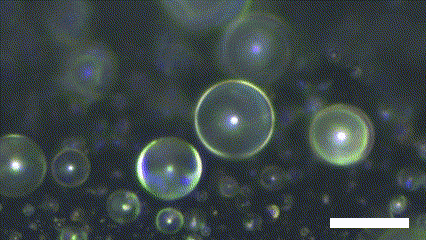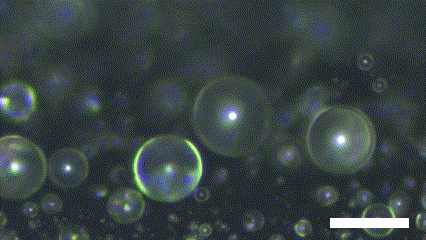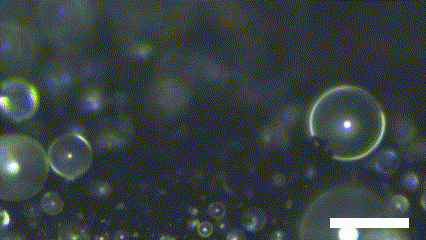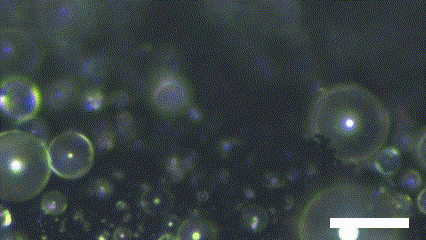
Superhydrophobic surface possesses a novel characteristic of coalescence-induced jumping, which is the phenomenon of the self-propelling of the coalescence droplet (Figure 2). There is a decrement in the total surface area of the droplets involved in the coalescence. It leads to a fraction of the surface energy in the initial system becoming excessed. The excess surface energy is converted to viscous energy, adhesion energy, and kinetic energy. Because the superhydrophobic surface has extremely low interaction with the water molecule, the energy converted to adhesion energy is low enough to provide enough kinetic energy to the resultant droplet. As a result, the asymmetry of the solid surface makes the resultant droplet propelled in the out-of-plane direction.
How does the coalescence-induced jumping matter?
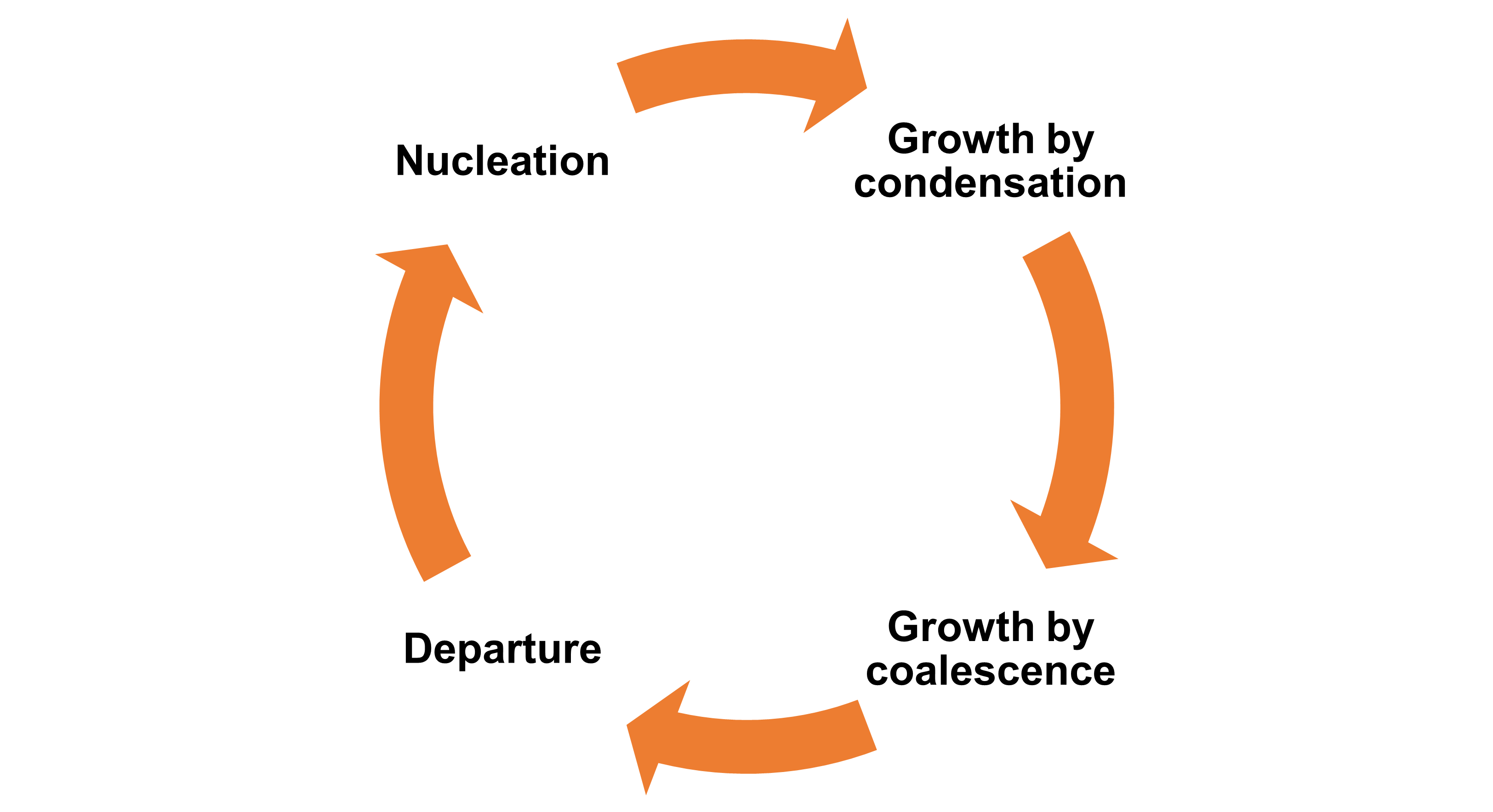
The coalescence-induced jumping provides an alternative departure mechanism to the droplet. For the conventional hydrophilic or hydrophobic surface, the droplet departure mechanism is gravity-driven, which has a limitation at the capillary length (~2.7mm). Whereas the droplet departure powered by excess surface energy has the critcial departure size at the micro-meter scale.
As a result, the droplets available in coalescence-induced jumping have a more efficient condensation cycle than the droplets depending on the gravity-driven departure. Moreover, it is commonly agreed that the superhydrophobic surface providing coalescence-induced jumping is more efficient than the hydrophilic and hydrophobic surface in terms of heat transfer2.
References:
- Cassie, A. B. D., & Baxter, S. (1944). Wettability of porous surfaces. Transactions of the Faraday society, 40, 546-551.
- Miljkovic, N., Enright, R., Nam, Y., Lopez, K., Dou, N., Sack, J., & Wang, E. N. (2013). Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano letters, 13(1), 179-187.